Abstract
Background
Bone marrow (BM) is an essential hematopoietic stem cell (HSC) source for many allogeneic transplants (HCT) and is the preferred cell source for specific disease indications in adults (e.g., Aplastic Anemia), the majority of pediatric transplants, and when the benefits of a decreased risk of chronic graft versus host disease (GVHD) outweigh other considerations. The advent of G-CSF mobilized peripheral blood stem cells (PBSC), with logistic benefits over the collection of BM, has resulted in a significant decrease in the utilization of BM. Since the advent of PBSC, BM has declined from 100% in the early 1990s to 15% in 2015, of unrelated donor products. This decreased utilization of BM has a number of potential consequences from a quality perspective. Many centers perform a limited number of BM collections per year and thus, the ability to ensure ongoing competency of staff in this technical procedure can be challenging. Proper assessment of BM harvest metrics is difficult in an individual collection facility when limited procedures are performed. Previous data from a single center noted that the total number of nucleated cells (TNC) received in BM products for individual patients decreased over the years. The aim of this study was to examine the trends in BM harvest quality in a large cohort of NMDP donors to see if this observation can be generalized.
Methods
The primary outcome of this study was examination of the total nucleated cells collected per milliliter (TNC/mL) of BM as an estimate of product quality. The population studied was BM donations from domestic unrelated donors reported to the NMDP/CIBMTR from 1994-2016 (analyzed over 5 time periods). The TNC/mL for each harvest was calculated based on the total number of nucleated cells (TNC) collected in harvest and the volume (mL) of final product including additives. Additional covariates, including time period of harvest, the number of harvests performed per year at the center, donor age, donor body mass index (BMI), donor weight, donor race/ethnicity, and donor gender were examined.
Results
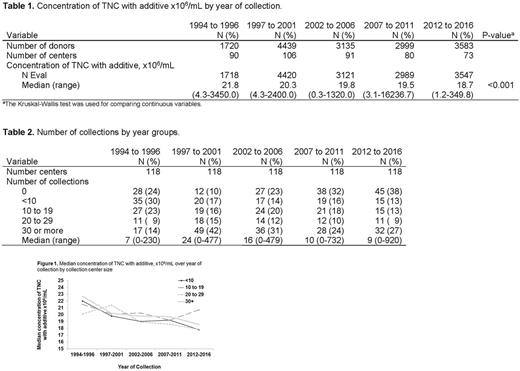
Examination of >15,000 BM donations revealed a significant decline in the concentration of total nucleated cells from 21.8 TNC x 106/mL in the earliest era to 18.7 TNC x106/mL in the 2012-2016 era (p<0.001). (Table 1). The number of overall collections performed per center in the 2012-2016 era was similar to other eras (Table 2). The number of collections performed by a collection center did not appear to explain the decrease in BM quality as median TNC/mL decreased over time intervals regardless of collection centre size (Figure 1). Interestingly, the total product volume harvest increased over the eras (p<0.001), while the duration of the harvest decreased (p<0.001). Donor characteristics were examined across eras. In recent years, donors are significantly more likely to be male (p<0.001) and young (p<0.001). For example, in the 2012-2016 era, 54% of the donors were under the age of 30 as compared to the 1994-1996 and 1997-2001 eras where only 25% of the donors were under the age of 30. Similarly, 65% of the donors were male in the 2012-2016 era, compared to 53% in the 1994-1996 era.
Conclusion
In conclusion, we showed a significant decrease in the TNC/mL in BM products over time. This did not appear to be related to the number of collections per center, suggesting that cell number collected is not directly related to the number of procedures performed but rather other factors in the collection process. Interestingly, the significant decrease in TNC concentration in BM observed in this study persisted despite the increased use of optimal donors. Further analysis will be required to elucidate the exact cause of decreased concentration of TNC collected in products. Factors such as technique, rate of collection, materials /equipment used, training, and collector experience may have profound influences on the quality of BM collected. For example, the number of personnel performing harvests, number of harvests performed per collector, or turn-over of personnel at each collection center maybe contributing factors. These further studies will help to better understand the entire process and help improve the quality of the products, an issue crucial to ensuring bone marrow remains a quality cell source for transplant.
Pulsipher: Jazz Pharmaceuticals: Other: advisory board, education; CSL Behring: Other: advisory board Feb 2017; Adaptive: Other: Advisory board 6/17; Chimerix: Other: Advisory board Dec 2015; Novartis Pharmaceuticals Corporation: Consultancy, Other: Phase II steering committee.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal